In this article:
Vaccination is an important element of public health, protecting individuals and communities from various contagious infections.
Women, in particular, have unique vaccination needs due to their reproductive health and anatomy. Vaccinations help prevent diseases that can lead to substantial damage to women’s health. (1)
Immunity can be actively achieved by utilizing substances that trigger an immune response, such as vaccines or modified toxins, or passively acquired through the introduction of antibodies such as immune globulins or antitoxins.
A modified toxin, known as a toxoid, retains its ability to stimulate antibody production while being rendered harmless. A vaccine is a solution containing inactivated or fractionated bacteria or viruses that have been made nonharmful. (1)
It is crucial to adhere to the recommended administration guidelines stated in the vaccine package insert to ensure optimal effectiveness.
Important Vaccines for Women
Here are some important vaccines for every woman, including their names, importance, side effects, frequency, age, and other important information.
1. Human papillomavirus (HPV) vaccine
The human papillomavirus (HPV) vaccine is capable of preventing the strains of the virus that are responsible for the majority of cervical cancers, as well as some cancers affecting the anus, vulva (the area surrounding the vaginal opening), vagina, and oropharynx (the back of the throat, including the base of the tongue and tonsils).
The HPV vaccine is essential for every woman since it can protect against several types of cancer. (2)
Who needs it?
The HPV vaccine is advised for girls aged 11–12 years, with a follow-up vaccination instructed for females aged 13–26 years who have not been immunized earlier. (3)
Safety
The Food and Drug Administration (FDA) has granted a license to the HPV vaccine, which has been deemed safe and adequate by the Centers for Disease Control and Prevention (CDC).
Clinical trials of the vaccine involving thousands of individuals from across the globe revealed no significant safety issues. The reported side effects from these trials were generally mild, such as localized pain at the injection site, fever, dizziness, and nausea. (2)
Contraindication: Is it safe for pregnant women to receive the HPV vaccine?
The HPV vaccine is not advised during pregnancy. While studies suggest that the vaccine does not pose any risks to the unborn child, more research is required to confirm this.
Women should avoid receiving any doses of the HPV vaccine until after the completion of their pregnancy. (4)
2. Influenza vaccine
It’s important for pregnant women to get vaccinated against influenza (flu). It can make you really sick, particularly when you’re pregnant or just had a child. It can even lead to pneumonia, which is a serious lung infection.
So, getting the flu shot is a crucial part of taking care of yourself and your baby before, during, and after pregnancy.
Who needs it?
According to the CDC, the influenza vaccine should be administered to all individuals (6 months and older) every year before/during the flu season.
It is more important for those who are pregnant, are planning to become pregnant, or have a weak immune system.
Pregnant women can receive the influenza vaccine during any trimester of pregnancy, and the antibodies can be passed to their newborns, protecting them from the flu as well. (5)(6)
Safety
The influenza vaccine has been associated with the following adverse events: (6)
- Injection site reactions
- Fever
- Irritability
- Drowsiness
- Myalgia (muscle pain or aches)
Contraindications
You should not get the influenza vaccine without talking to a doctor first if you have the following: (6)
- A record of allergy or hypersensitivity to any ingredient of the vaccination (such as an allergy to egg protein)
- Age younger than 6 months
- High fever
- Guillain–Barre syndrome (a rare neurological disorder)
3. Tetanus, diphtheria, and pertussis (Tdap) vaccine

The Tdap vaccine is highly recommended for every woman, especially those who are pregnant or planning to become pregnant. The Tdap vaccine protects from tetanus, diphtheria, and whooping cough. (7)(8)
Who needs it?
The vaccine is typically given during the gestation period to women between 27 and 36 weeks of pregnancy.
After childbirth, the Tdap vaccine is administered routinely to the children:
- A single dose for adolescents aged 11–12 years.
- If the initial dose was missed, a dose can be given at a later age.
- For grownups who have not obtained a Tdap vaccine previously, a single dose is given.
- A single dose is given to children who have not completed their primary DTaP vaccine series.
The vaccine is also recommended for adults who have not received the Tdap vaccine previously. (7)
Safety
After receiving the Tdap vaccine, common adverse effects may include pain, redness, or edema at the vaccination site, mild fever, headache, fatigue, and gastrointestinal symptoms such as diarrhea and stomachache. (7)
Contraindications
If you have conditions such as infantile spasms or uncontrolled epilepsy, you can still receive the vaccine, but extra care and monitoring may be needed. (9)
4. Measles, mumps, and rubella vaccine
The MMR vaccine safeguards against measles, mumps, and rubella, which are serious viral diseases transmitted through contact with droplets from infected individuals.
Measles causes a rash, fever, and other complications, mumps affects the glands, and rubella can cause birth defects in infants if contracted by pregnant women. (10)
Who needs it?
The CDC advises administering 2 doses of the MMR vaccine to all children, with the first dose given between 12 and 15 months of age and the 2nd dose between 4 and 6 years of age.
Women who have not yet been vaccinated against measles, mumps, and rubella and have poor immunity should receive at least one dose of MMR vaccine before becoming pregnant or during the postpartum period.
Breastfeeding women can safely receive the MMR vaccine without affecting their response to the vaccine or their baby. (11)(12)
Note: Pregnant individuals should not receive live-virus vaccines such as MMR and chickenpox, but they can receive these vaccines before or after pregnancy, if necessary. (13)
Safety
In general, adverse events are more common after the first dose, and about 5% of vaccinated children may experience malaise (feeling of illness) and fever, with or without a rash, that lasts up to 3 days, within 1 to 3 weeks after vaccination. (11)
Contraindications
Some people may need a doctor’s advice before getting this vaccination, such as those with severe allergies or weakened immune systems or those who have gotten other vaccines very recently.
Also, pregnant women should get it only after childbirth after talking with their healthcare provider. (11)(12)
5. Hepatitis B vaccine
The hepatitis B vaccine is a medicine that helps prevent a disease called hepatitis B, which is caused by a virus. This disease can harm the liver and even cause liver cancer if it becomes a long-term problem.
The hepatitis B vaccine is suggested for every woman, especially those who are in increased danger of contracting the virus. (14)
Who needs it?
Everyone needs to get this vaccine. It is given in three doses with the first dose given, ideally, within 24 hours of birth. The second dose is given after a month, and the third dose 6 months after the second.
Boosters or other doses are not required. You only need these three-dose series of hepatitis B vaccination across 6 months, and you can get them at any age if you did not get them as an infant. (15)
This vaccination can also be taken during any stage of pregnancy and breastfeeding if you did not receive them during infancy. (16)
Note: It is not generally required to get vaccinated again during pregnancy if you already have received all three doses of hepatitis B vaccination before.
So, it is never too late to get vaccinated against hepatitis B for lifelong protection against this chronic liver disease.
Safety
Studies have shown that the hepatitis B vaccine is safe for people of all ages. In the United States, more than 70 million people obtained at least one dose of the vaccine from 1982 to 2004. The most generally documented adverse effects were pain at the injection site and a temperature above 99.9 °F. (14)
Contraindications
People who have had an allergic reaction to any part of the hepatitis B vaccine or who are allergic to yeast should not receive the vaccine. (14)
6. Varicella (chickenpox) vaccine
Chickenpox is an infectious disorder caused by the varicella-zoster virus. It can stay in the body after the initial infection and reactivate later in life, causing a condition called shingles.
The varicella vaccine is recommended for every woman who has not been previously vaccinated or has not had chickenpox. (17)(18)
Who needs it?
The vaccine is typically given in two doses, with the first dose advised at 12–15 months of age and the second dose recommended at 4–6 years of age.
In pregnant women, vaccination for chickenpox is not recommended. Women should wait for 3 months after getting the vaccine before trying to get pregnant. (18)
Note: If you received the recommended two doses of the chickenpox vaccine in childhood, it is unlikely that you will need to get vaccinated again as an adult, even if you become pregnant.
Safety
After getting the varicella vaccine, you might have a sore arm, redness, rash, or fever. These are common side effects.
Serious reactions are very rare. These may include pneumonia, infection of the brain or spinal cord covering, or seizures with fever. (17)
Contraindications
The varicella vaccine should not be delivered to people who are severely allergic to neomycin or gelatin or have had an intense response to an earlier dose of the vaccine.
It should also not be given to people with weak immune systems, such as those with AIDS or cancer, or those receiving treatments such as chemotherapy or steroids.
Additionally, it is not recommended for people with a family history of weak immune systems unless they have been shown to have a strong immune system themselves.
Pregnant individuals should not receive live-virus vaccines such as MMR and chickenpox, but they can receive these vaccines before or after pregnancy, if necessary. (13)(18)
7. Meningococcal vaccine
The meningococcal vaccine is recommended for women, especially those who are college students or live in close quarters with others. The vaccine safeguards against meningococcal disease, which can cause meningitis and sepsis. (19)
Who needs it?
The meningococcal vaccine is typically given between the ages of 11 and 12 years, with a follow-up dose recommended at age 16 years. (20)
College students and individuals with weakened immune systems should also receive the vaccine if they haven’t been vaccinated before or if it has been more than 5 years since their last dose. (20)
In some cases, healthcare providers may recommend a booster dose of the meningococcal vaccine during pregnancy to ensure adequate protection for both the mother and the baby. (21)
Safety
Clinical trials and international reporting systems have not reported any significant adverse reactions to the vaccine. Most of the side effects were mild and related to the injection site, such as redness, swelling, and pain. Headaches, dizziness, and fever were less commonly reported after vaccination. (22)
Contraindications
Those who have had an allergic response to any component of the meningococcal vaccine should not receive it. Similarly, those who have had a severe allergic reaction to vaccines containing diphtheria or tetanus toxoids should not receive meningococcal vaccines that are conjugated with these toxoids. (22)
8. Pneumococcal vaccine
The pneumococcal vaccine safeguards against pneumococcal disorder, which can result in pneumonia, meningitis, and other illnesses.
Chronic heart disease, chronic lung disease (including asthma), diabetes mellitus, cerebrospinal fluid leak, cochlear implant, alcohol use disorder, chronic liver disease, and cigarette smoking are conditions that may indicate the need for a pneumococcal vaccine. (23)
Who needs it?
The pneumococcal vaccine is recommended as a routine childhood vaccination at specific intervals. Adults with risk factors such as heart disease, lung disease, diabetes mellitus, cochlear implants, alcohol use disorder, chronic liver disease, and cigarette smoking should also receive this vaccine.
Moreover, the pneumococcal vaccine is especially recommended for those who are over the age of 65 years even if they have gotten it before in childhood.
If you have received the pneumococcal vaccine in childhood and you are now pregnant, it is generally not necessary to repeat it, but if you’ve not received it before, this vaccination is safe to get during pregnancy and is recommended for those at a high risk of infection. (23)
Safety
Adverse effects have been reported for pneumococcal vaccines in different age groups. Some of the most common adverse effects are:
- Infants and toddlers: Irritability, tenderness, reduced appetite, reduced sleep, fever, skin redness, injection site edema
- Children aged 5 to 17 years: Tenderness, redness, swelling, irritability, reduced appetite, reduced sleep, fever
- Adults 18 years and older: Pain, fatigue, headache, muscle pain, joint pain, reduced appetite, redness, edema, restriction of arm movement, emesis, fever, chills, rash. (23)
Contraindications
The vaccine should not be given to people who have had an extreme response or anaphylactic reaction to any ingredient of the vaccine formulation or any vaccine containing diphtheria toxoid. (23)
9. Shingles vaccine
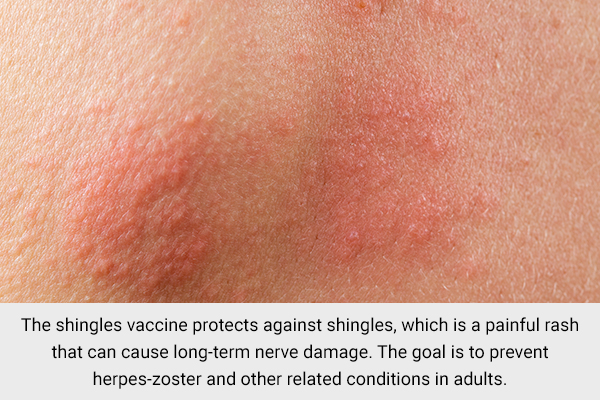
The shingles vaccine protects against shingles, which is a painful rash that can cause long-term nerve damage. The goal is to prevent herpes-zoster and other related conditions in adults. (24)
Who needs it?
The shingles vaccine is advised for every female over the age of 50 years. It is given in two doses, with the second dose given 2–6 months following the first dose.
The vaccine is also advised for people who have had shingles before. (24)(25)
Safety
In some studies, people aged 50 years and above reported some discomfort after getting two doses of the vaccine. They had pain, redness, and edema in the region where they got the vaccine. They also felt tired and had headaches, and some had a fever or upset stomach.
Those who were 50 to 69 years old had more of these reactions than those who were 70 years or older. Most of these reactions lasted for about 2–3 days. (26)
Contraindications
People who have had a severe allergic response to any part of the vaccine should not get this shot. It’s also not advised for pregnant or breastfeeding women, as well as for those who have a severely weak immune system, even though it’s not prohibited. (26)
General Risks, Restrictions, and High-Risk Groups
Here are some points to remember when getting vaccinated:
- If patients are undergoing short-term immunosuppressive therapy, live-virus vaccines should be delayed until after the therapy is completed.
- Patients on long-term immunosuppressive treatment may obtain inactivated vaccines and can then obtain live-virus vaccines 3 months after ceasing immunosuppressive treatment. (27)
- It is important to avoid giving live-microbial vaccines at the same time as blood, plasma, or immune globulin, as these can hamper the required antibody response. Ideally, such vaccines should be given 2 weeks before or 6–12 weeks after immune globulins.
- Patients without a spleen are in danger of severe bacterial infections and should obtain the Hib conjugate vaccine, meningococcal polysaccharide vaccine, annual influenza vaccine, and pneumococcal conjugate or polysaccharide vaccine. (28)
- Before solid organ transplantation, patients should obtain all applicable vaccines, and those who have undergone hematopoietic cell transplantation should obtain repeat doses of all applicable vaccines. (29)
- Patients with AIDS should commonly obtain inactivated vaccines according to routine guidance but should bypass live-virus or bacterial vaccines, although the MMR vaccine may be assessed if immunosuppression is not intense. (30)
Vaccination for Pregnant Women
Here are some points to keep in mind about vaccination in pregnant women as shared by various experts and researchers: (31)
- Pregnant women should not get live-virus vaccines.
- Pregnant women should not get MMR, pneumococcal, varicella, or HPV vaccine.
- It’s safe for pregnant women to get the Tdap, flu, and tetanus toxoid vaccines.
- If you are pregnant during the influenza season, then you should get the influenza vaccine.
- MMR vaccine should be given before pregnancy, and it’s advised to avoid pregnancy for a month after the vaccine.
- In the third trimester, all pregnant females should get Tdap and TT vaccines.
- Shots for hepatitis A and B, pneumococcal, meningococcal, yellow fever, Japanese encephalitis, polio, typhoid, and cholera infections should be offered to women with an increased risk of these infections. (31)
- There have been some talks about how thimerosal, a common ingredient found in vaccines, can be unsafe for infants. But there is a lack of research, and manufacturers do provide such vaccines to infants too.
- If a patient has a progressive neurological condition, they should not be immunized till they have been stable for at least 1 year because of the danger of cerebral irritatio.n
- Smallpox, rabies, and anthrax shots should just be given for post-exposure prophylaxis (PEP).
- Rh-negative pregnant women receive Rh0(D) immune globulin 300 μg IM after vaginal bleeding, abortion, or prenatal tests, at 28 weeks, and after delivery if the baby has Rh0(D)-positive blood.
- Postpartum women should get an HPV shot, and if not vaccinated before, they should get MMR, Tdap, and Flu shots. (31)
Most-Asked Questions About Vaccines for Women
Are vaccinations safe for pregnant women?
Yes, most vaccinations are safe for pregnant women, and some are even recommended to protect both the mother and the unborn child.
Women should talk with their healthcare providers before receiving any vaccines during pregnancy. (31)
Can vaccines lead to autism?
No, vaccines do not result in autism. Multiple studies have shown that there is no association between vaccines and autism. (32)
Can vaccines cause severe allergic reactions?
Severe allergic reactions to vaccines are rare but can occur. Individuals should discuss their vaccination history and any allergies with their healthcare providers before receiving any vaccine.
Healthcare providers can take necessary precautions to prevent severe allergic reactions. (33)
Can I still get the disease even after getting vaccinated?
While vaccines are positively useful, no vaccine delivers 100% defense against a disease. Nonetheless, even if you do suffer from the infection after being immunized, the vaccine can decrease the stringency of the disease and prevent complications. (34)
Can I get vaccinated if I have a cold or flu?
If you have a temperate ailment, such as a cold or flu, you can still receive most vaccines. Nonetheless, if you have a fever or a more harsh disease, you should hold off until you heal before getting immunized. (35)
What should I do if I miss a vaccine dose?
If you miss a vaccine dose, you should talk to your healthcare provider about when you should receive the next dose.
Can I get vaccinated if I have a history of allergic reactions to vaccines?
If you have a history of severe allergic reactions to vaccines or vaccine components, you should talk to your healthcare provider about your vaccination options.
In some cases, individuals with a history of severe allergic reactions may not be able to receive certain vaccines.
Final Word
Vaccinations are essential for every woman, protecting them from various infectious diseases that can cause significant harm to their health and well-being.
Women should discuss their vaccination needs with their healthcare providers and follow the recommended vaccination schedules. By getting vaccinated, women can protect themselves, their families, and their communities from preventable diseases.
- Was this article helpful?
- YES, THANKS!NOT REALLY


